银屑病关节炎和非放射性轴性脊柱关节炎都是慢性炎症性疾病。银屑病关节炎可导致无法逆转的关节变形和残疾。据估计,全球有超过5000万名银屑病关节炎患者。轴性脊柱关节炎(axSpA)则主要影响骶髂关节和轴性骨骼,全球有大约450万成年患者。其中,nr-axSpA通常难以被诊断,很多患者较难获得妥善的治疗。
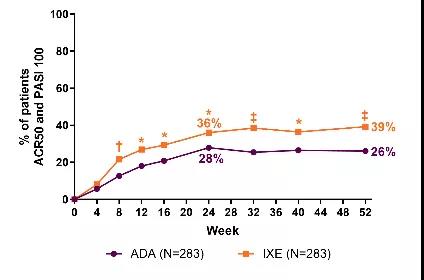
礼来公司的单克隆抗体Taltz,可选择性结合白细胞介素17A(IL-17A),并抑制其与IL-17受体的相互作用。IL-17A是一种天然存在的细胞因子,参与正常的炎症和免疫反应。通过抑制IL-17受体介导的信号通路,Taltz能抑制促炎细胞因子和趋化因子的释放,从而缓解炎症性疾病的症状。此前,Taltz已获批用于治疗活跃性PsA,活跃性强直性脊柱炎(AS),以及适合进行全身治疗或光疗的中重度斑块状银屑病成人患者。

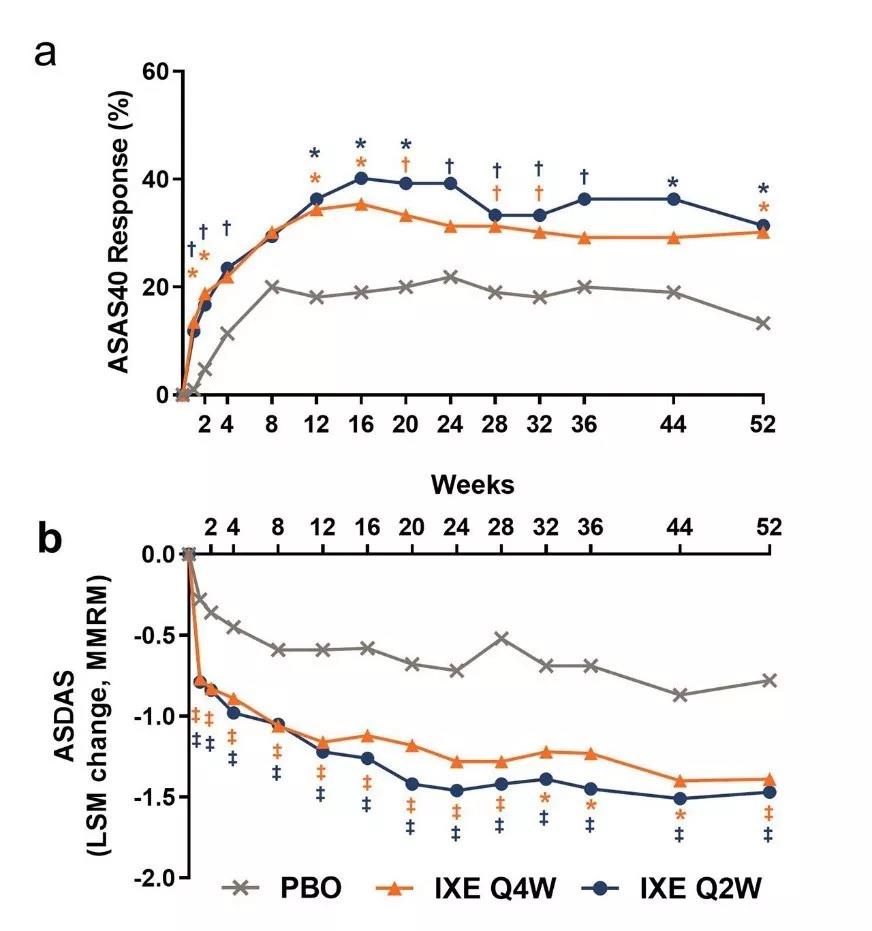
在这项名为COAST-X的3期临床试验中,尚未接受过生物制剂类抗风湿药物(bDMARD)治疗的nr-axSpA患者接受了Taltz或安慰剂的治疗。试验数据表明,治疗52周后,治疗组中达到脊柱关节炎国际协会评定40(ASAS40)的患者比例分别为30%(每4周接受一次治疗)和31%(每2周接受一次治疗),而在安慰剂组中的数值为13%。
参考资料:
[1] ACR 2019: Lilly Presents 52-Week Head-to-Head (SPIRIT-H2H) Data from Taltz® (ixekizumab) Versus Humira® (adalimumab) Trial in Psoriatic Arthritis, Retrieved November 12, 2019, from https://investor.lilly.com/news-releases/news-release-details/acr-2019-lilly-presents-52-week-head-head-spirit-h2h-data-taltzr
[2] ACR 2019: Lilly Presents Positive New Data from COAST-X, a Phase 3 Study of Taltz® (ixekizumab) in Patients with Non-Radiographic Axial Spondyloarthritis,Retrieved November 12, 2019, from https://www.prnewswire.com/news-releases/acr-2019-lilly-presents-positive-new-data-from-coast-x-a-phase-3-study-of-taltz-ixekizumab-in-patients-with-non-radiographic-axial-spondyloarthritis-300954995.html
[3] A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks,Retrieved November 12, 2019, from https://acrabstracts.org/abstract/a-head-to-head-comparison-of-ixekizumab-and-adalimumab-in-biologic-naive-patients-with-active-psoriatic-arthritis-efficacy-and-safety-outcomes-from-a-randomized-open-label-blinded-assessor-study-th/
[4] Ixekizumab in Non-Radiographic Axial Spondyloarthritis: Primary Results from a Phase 3 Trial,Retrieved November 12, 2019, from https://acrabstracts.org/abstract/ixekizumab-in-non-radiographic-axial-spondyloarthritis-primary-results-from-a-phase-3-trial/

