
此前,该药物曾在美国进行的一项2期临床研究中接受检验,该研究名为EVIDENCES IV,是一项随机,双盲,含安慰剂对照组的研究,共有106名患者参与。该试验的主要研究终点是在第16周时,与安慰剂组相比,接受saroglitazar magnesium治疗患者的肝脏丙氨酸转氨酶(ALT)水平较基线时的变化。试验的次要终点包括通过无创核磁共振质子密度脂肪分数(MRI-PDFF)测量的肝脂肪含量变化等多项指标。试验结果表明,与安慰剂组相比,saroglitazar magnesium使患者的ALT水平下降了44.39%。此外,通过MRI-PDFF定量评估患者的肝脂肪含量也达到了统计学意义上的显著降低。
参考资料:
[1] Zydus Announces World's First Drug for the Treatment of Non-Cirrhotic NASH, Retrieved March 05, 2020, from https://www.prnewswire.com/news-releases/zydus-announces-worlds-first-drug-for-the-treatment-of-non-cirrhotic-nash-301017439.html
[2] Zydus Announces Positive Results From EVIDENCES IV Phase 2 Clinical Trial of Saroglitazar Magnesium in NAFLD and NASH. Retrieved March 05, 2020, from https://www.biospace.com/article/releases/zydus-announces-positive-results-from-evidences-iv-phase-2-clinical-trial-of-saroglitazar-magnesium-in-nafld-and-nash/
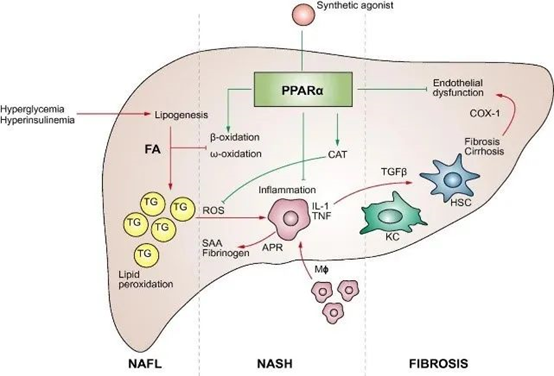
[3] Pawlak et al., (2015). Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology, https://doi.org/10.1016/j.jhep.2014.10.039

