以下文章来源于:药明康德
今日,IDEAYA Biosciences公司宣布,其基于“合成致死”原理开发的小分子MAT2A抑制剂IDE397,在1/2期临床试验中获得积极结果。早期结果显示,在接受高剂量IDE397治疗的患者队列中,75%的患者循环肿瘤DNA(ctDNA)水平显著下降,获得分子生物学缓解。该公司同时宣布与安进(Amgen)公司达成合作,将评估IDE397与安进的PRMT5抑制剂AMG 193联用,治疗MTAP缺失肿瘤的效果。

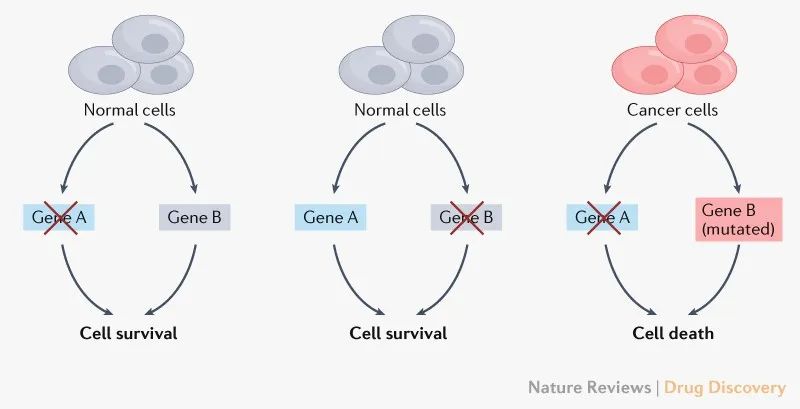
▲合成致死用于治疗癌症的理念(图片来源:参考资料[1])
参考资料:
[1] IDEAYA Announces IDE397 Clinical Program Update and ctDNA Molecular Responses Demonstrating Tumor Pharmacodynamic Modulation. Retrieved July 27, 2022, from https://www.prnewswire.com/news-releases/ideaya-announces-ide397-clinical-program-update-and-ctdna-molecular-responses-demonstrating-tumor-pharmacodynamic-modulation-301594078.html
[2] IDEAYA Announces Clinical Trial Collaboration with Amgen to Evaluate MAT2A-PRMT5 Synthetic Lethality Combination in MTAP Deleted Tumors. Retrieved July 27, 2022, from https://media.ideayabio.com/2022-07-27-IDEAYA-Announces-Clinical-Trial-Collaboration-with-Amgen-to-Evaluate-MAT2A-PRMT5-Synthetic-Lethality-Combination-in-MTAP-Deleted-Tumors
[3] Mullard. (2022). What’s next for the synthetic lethality drug discovery engine? Nature Reviews Drug Discovery, doi: https://doi.org/10.1038/d41573-022-00107-0

